|
 |
|||||||||||||
|
|||||||||||||
|
|||||||||||||
|
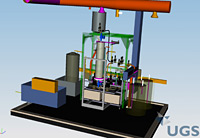
To make the superconducting cavities for the ILC sparkle, they must undergo a series of surface treatments to make them as clean and pure as possible – a necessity for achieving high accelerating gradients. Electropolishing and Buffered Chemical Polishing, the two types of chemical treatments required for the cavities, are not simple tasks. They involve tricky chemicals and a detailed recipe for producing the best cavities possible. For some time now, physicists around the world have studied the benefits of these treatments, trying to define the optimal recipe for superconducting cavities, at state-of-the-art facilities at DESY, KEK, JLab and Cornell University. In fact, breakthrough developments at these facilities helped prove that electropolishing vastly improves acceleration gradients. Work continues at these facilities to study how a combination of electropolishing and BCP may be the ticket for consistently achieving a high acceleration gradient for the ILC. (see ILC NewsLine, 29 June 2006) Last April, Argonne and Fermilab signed a Memorandum of Understanding to join the ongoing international effort and collaborate on a superconducting cavity processing facility for ILC cavity R&D. To help push ILC cavity processing capabilities in the US forward, the two laboratories created a new Joint Superconducting Surface Processing Facility infrastructure, located at Argonne, with the capability to perform both buffered chemical polishing and electropolishing procedures on ILC cavities. In December, Fermilab engineer Allan Rowe provided an update on the design and commissioning of the facility's new BCP system for processing 1.3 GHz cavities for the ILC. "The BCP System was designed with an eye toward future industrialisation of the BCP process," Rowe said. "The design and layout of the BCP System combines many prudent operational and safety features of other facilities currently performing BCP procedures. We hope the system at the SCSPF will be a step in the right direction toward developing a repeatable process we can pass along to industry." Nothing like your mom's polish for the good silver, BCP consists of a 1:1:2 ratio of concentrated nitric, hydrofluoric, and phosphoric acids. This acidic and potent cocktail literally polishes both the inner and outer surfaces of the cavity by removing the damaged layer of impurities within the niobium material. Each acid in the mix serves a specific purpose in the BCP process. The nitric acid reacts with the niobium to form an oxide layer, which is then dissolved by the hydrofluoric acid. The freshly exposed niobium then reacts with the nitric acid. This cycle repeats rapidly, releasing significant heat. If the reaction occurs at too high a temperature, hydrogen, a byproduct, diffuses into the niobium and lowers the cavity's Q (quality) factor, a measure of the material's resistance. Therefore, to keep the reaction rate constant, hence lowering the heat generation rate, phosphoric acid serves as a buffer. Scientists are still writing the perfect recipe for how to best chemically treat a superconducting cavity for the ILC. Repeatability is of the utmost importance and further R&D will better define the processes. When operating at a temperature of 10 to 12 degrees Celsius, current processes call for the acid to flow through the cavity at an etching (or polishing) rate of approximately one micron per minute. When treating the outside surfaces of the cavity, approximately 20 microns of the outer surface need to be removed. A thicker layer of at least 80 microns must be removed from the current and magnetic field carrying inside surfaces. If the cavity will also undergo an electropolishing treatment, however, only the outer surfaces get chemically polished. Even though electrons and positrons will travel inside the cavities in the ILC, treating the outer surfaces of these niobium structures is important. The same contaminants that exist inside the cavity also exist on the outside, making it possible for them to migrate and limit the acceleration gradient. When designing a BCP system, safety is the top priority. Fermilab's engineers integrated a number of safety features into the BCP system. Some safety features include: a ventilation system that eliminates technicians' fume exposure; an automated neutralisation system that processes dilute acid waste; and a remote control center that uses a Programme Logic Controller to control all BCP System operations. BCP System operators will monitor the polishing procedures from the control center located outside the processing room. The BCP system will go through a complete safety review before the joint Argonne-Fermilab facility can be used to process cavities. All of these automated systems keep technicians' exposure and handling of the chemicals to an absolute minimum. "We have a very rigid safety program," Rowe said. "Once the system is approved for use, we will have highly-trained operators and an accompanying facility capable of safely performing chemical polishing procedures on ILC cavities near Fermilab." Rowe expects to begin commissioning the BCP system with water in January and a safety review to follow this spring. -- Elizabeth Clements |
|||||||||||||
| © International Linear Collider |